Iron Porphyrins and Copper Nanocubes Team Up to Enhance Ethylene Production in Electrocatalytic CO2 Reduction
Press release
Scientists at EPFL, ICIQ and TUM have built new hybrid materials based on molecular Fe porphyrins and Cu nanocubes that act in tandem for the electrocatalytic reduction of CO2 to ethylene. The modular nature of this hybrid materials opens up facile and versatile routes towards the preparation of this type of hybrid materials that can further enhance the CO2 reduction efficiency and selectivity.
Lausanne (Switzerland), Munich (Germany) and Tarragona (Spain), November 26th 2022 – The EU-funded LICROX project aims to develop a new type of photoelectrochemical cell (PEC) to transform sunlight, water and CO2 into carbon-based molecules capable of storing chemical energy. The LICROX design for the PEC combines different research layers to achieve the conversion of sunlight, water and CO2 into carbon-based molecules. Scientists at EPFL, TUM and ICIQ came together to develop a tandem catalytic system composed of a molecular catalyst, able to transform CO2 into CO, and copper nanocubes that allow carbon-carbon (C-C) coupling.
The electrochemical CO2 reduction reaction (CO2RR) offers the opportunity to convert CO2 into fuels and chemical feedstocks while mitigating anthropogenic CO2 emissions and storing renewable energy. Multi-carbon (C2+) compounds (e.g., ethylene, ethanol, propanol) are among the most attractive CO2RR products owing to their commercial value and high energy densities. However, optimizing the activity of CO2RR electrocatalysts towards one particular product remains a crucial challenge that is not trivial to address because multiple intermediates are involved in the CO2 to C2+ conversion pathway.
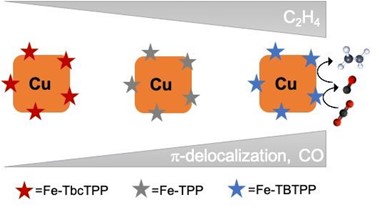
“Molecular Fe Porphyrins complexes constitute a tunable class of robust and extremely active CO2 reduction catalysts for the formation of CO, in a highly selective manner” says Prof. Antoni Llobet, ICIQ Group Leader, LICROX Coordinator
Copper-based materials have been widely employed as CO2RR catalysts due to their unique ability to facilitate the electroreduction of CO2 beyond two electron reduction products. In particular, copper nanocubes are particularly selective for C2+ .
In this recent publication, Buonsanti, Sharp and Llobet teams have worked together coupling the molecular Fe porphyrins and the copper nanocubes to further enhance the intrinsic selectivity of the copper towards multi-carbon products at lower overpotentials. This work demonstrates that the CO generated by the molecular catalyst is transferred to the copper when it is further converted in a so-called tandem scheme. A 22-times increase in ethylene selectivity and 100 millivolts positive shift of the onset potential was observed compared to the nanocubes alone.
This article is part of the 2022 Chemical Science HOT Article Collection.
“These results encourage further studies on the design of hybrid materials including molecules and well-defined nanoparticles as a promising strategy to create more active and selective catalysts for the electrochemical CO2 reduction reaction” says Prof. Raffaella Buonsanti, Associate Professor at EPFL, LICROX partner.
Reference article:
Min Wang, Vasilis Nikolaou, Anna Louidice, Ian D. Sharp, Antoni Llobet, Raffaella Buonsanti. Tandem electrocatalytic CO2 reduction with Fe-porphyrins and Cu nanocubes enhances ethylene production. Chem. Sci, 2022, 13, 12673.